Home » Lab Capabilities » Dissolution
Avivia Dissolution Testing Services

The experience and knowledge-based pharmaceutical dissolution development platform within Avivia focuses primarily on supporting formulation development and problem solving.
For us, dissolution testing is never a routine test, but an essential tool in pharmaceutical formulation development to:
- evaluate the physico-chemical properties of drug candidates to select the most appropriate solid form for further development (pre-formulation)
- perform biorelevant in-vitro tests to mimic fed and fasted state conditions
- compare prototype formulations (preclinical selection)
- elucidate drug release mechanism (diffusion, erosion, etc.)
- as an indicator of stability (e.g., temperature, humidity)
- as an indicator of the robustness of the manufacturing and
- assure safe release and reproducibility of the products to the market.
Dissolution testing should be a discriminatory method that is sensitive to variables affecting release rate and ideally has a predictive value for bio-performance.
It takes a lot of skills, knowledge and experience to design dissolution testing methods that contribute to the development of safe, robust and successful pharmaceutical dosage forms.

Avivia is an independent pharmaceutical development contracting organization in the Netherlands offering a unique, complementary range of pharmaceutical services in the areas of:

Dissolution Development
The Avivia dissolution development team has years of experience developing bio-relevant dissolution methods to support formulation development, for both generics, generic plus and new products, from BCS class I to IV drug substances, from immediate release tablet to parenteral depot formulations.
Read more about our unique dissolution development possibilities.
Dissolution Basics and Theory
Dissolution testing of pharmaceutical dosage forms is an important in-vitro test to provide critical in-vitro drug release information. The dissolution test is used in different stages, from early formulation development, drug product registration and the commercial phase:
1. A quality control tool
- Batch tot batch consistency
- Provide quality assurance
- The only test that can monitor if the rate of drug solubilization is impacted by drug product storage conditions
2. Dissolution has being identified as a surrogate for bioavailability
- Some manufacturing changes can be approved based only on the comparability of either their dissolution profiles without having to conduct in-vivo studies
3. Guide formulation development
- The only product test that truly measures the effect of formulation and physical properties of the API on the rate of drug solubilization.
The dissolution process can be described as a schematic process, the dissolution rate with the Noyes–Whitney equation.

Dissolution Apparatus Equipment
Within Avivia dissolution testing development services laboratory we have a complementary range of dissolution testing equipment available:
• USP 1 / apparatus I / apparatus 1 / Basket dissolution apparatus
• USP 2 / apparatus II / apparatus 2 / Paddle dissolution apparatus
• USP 3 / apparatus III / apparatus 3 / Reciprocating cylinder dissolution apparatus / BioDis
• USP 4 / apparatus IV / apparatus 4 / Flow through cell dissolution apparatus
• Pion µDiss Profiler (low volume in situ fiber optic UV monitoring dissolution and solubility system)
Intrinsic dissolution testing and other dissolution techniques
As intrinsic dissolution apparatus both the Pion µDiss Profiler and Wood apparatus are present. Specific flow cells for our flow through cell dissolution apparatus are used for apparent dissolution testing of powders, like granules and active pharmaceutical ingredients.
And if required, other less common or more academic dissolution testing techniques are accessible in our network, or we design a custom made dissolution setup.
But most important we know how and when to apply all these systems in support of formulation development, troubleshooting and optimization.
Dissolution Case Study: drug vs hypromellose release
The specific analytical methodologies from the Avivia excipient expertise center Excipia allows us to determine not only drug release, but also other relevant drug product characteristics, such as, for example, hypromellose (HPMC) release from a controlled release hypromellose matrix tablets.
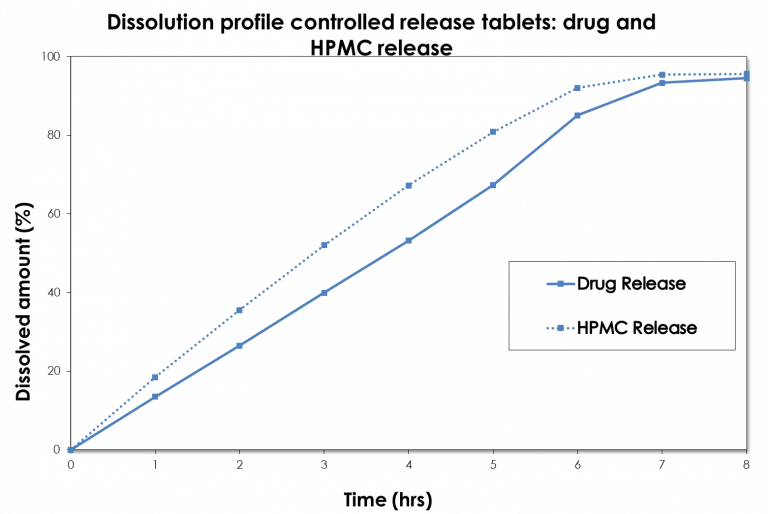
In this particular case, drug and HPMC release was monitored with the USP apparatus III (BioDis). The HPMC release was found to be faster than that of the drug itself; the release mechanism was primarily controlled by erosion. The diffusion-controlled release mechanism was almost absent, making the pharmaceutical formulation extremely sensitive to the HPMC properties. These properties were investigated extensively experts of Excipia and subsequently controlled by defining in-house specifications. Read more.
In cooperation with our clinical research partners we have successfully established in-vitro in-vivo correlations (IVIVC) for several modified release dosage forms.



